A recent Cell Reports paper by Grin et al. from the Overall Lab presents the first evidence of a viral protease being secreted from infected cells. 3C-like viral proteases (3CL) are utilized by coronaviruses, including severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), to cleave the viral polyprotein into individual functional proteins necessary for viral replication. In addition to these essential intracellular cleavage events, over 160 intracellular host substrates by the SARS-CoV-2 3CLpro (also known as main protease, Mpro) have been characterised and identified (Pablos et al. 2021, Cell Reports).
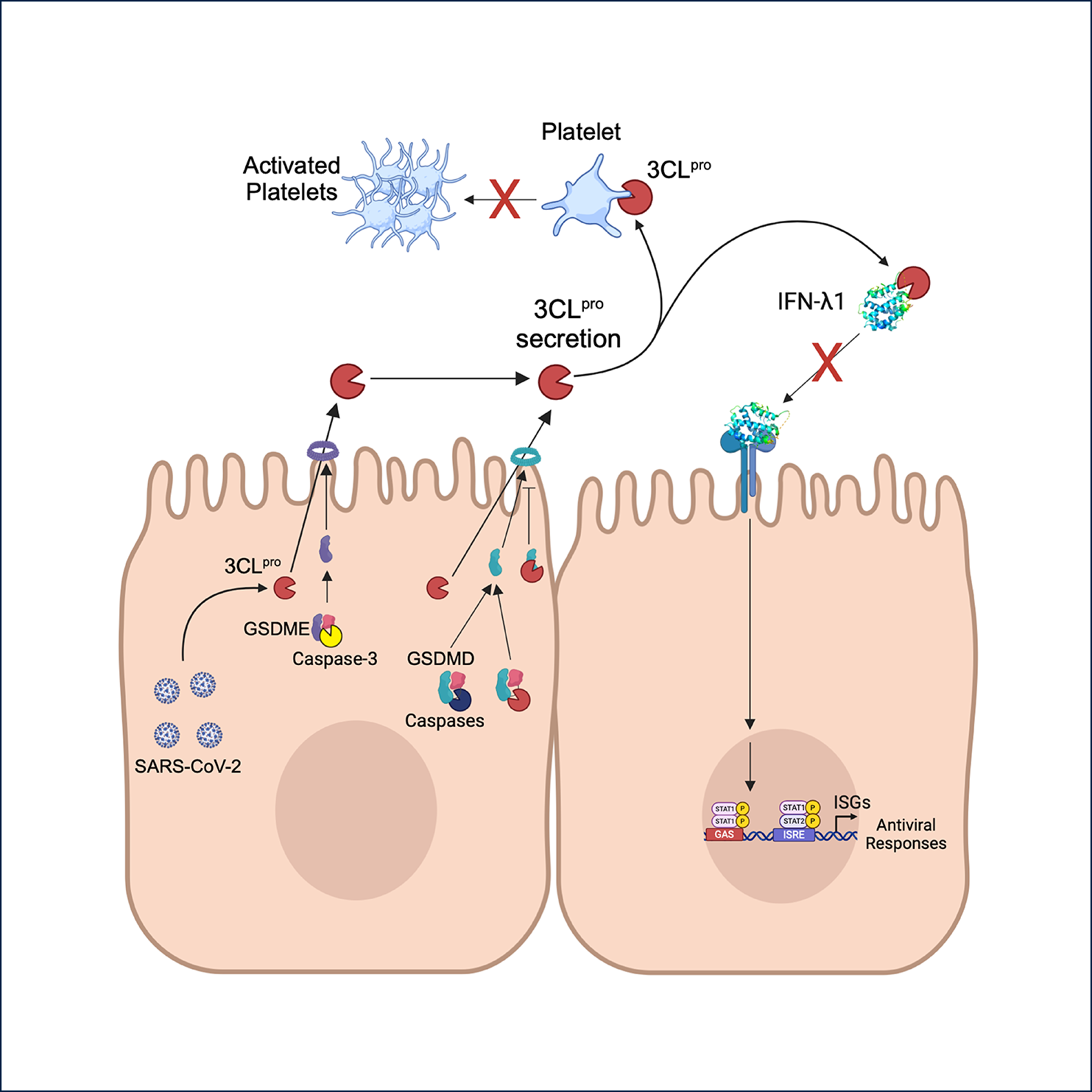
The researchers found that 3CLpro is secreted through gasdermin-D (GSDMD) and gasdermin-E (GSDME) pores, which are activated by caspases. These pores are the terminal effectors of pyroptosis—a form of cell death that is activated during infection. While these pores form conduits for 3CLpro and viral nucleocapsid protein release from infected cells, excessive pore formation kills the host cell by pyroptosis, inhibiting 3CLpro expression and secretion. To this end, 3CLpro performs a delicate balance: it regulates its own secretion through GSDMD pores by:
- It cleaves GSDMD at LH270↓N to activate pore formation, promoting its release.
- Subsequently, it cleaves at LQ29↓S and LQ193↓G to inhibit further pore formation, preventing excessive cell death.
By balancing the levels of pore formation to release 3CLpro but still maintain cell function by preventing pyroptosis, selected host and viral proteins are secreted by GSDMD and also GSDME pores.
Extracellular Functions of 3CLpro
Beyond its intracellular roles, 3CLpro exerts distinct functions in the extracellular environment. Researchers identified and characterized two inactivating cleavages in IFN-l1 by 3CLpro using amino terminal-oriented mass spectrometry. Such inactivation of IFN-l1 is hypothesized to be an immune escape mechanism for SARS-CoV-2.
Additionally, 3CLpro retained ~70% of its activity when incubated in human serum that contains high levels of endogenous protease inhibitors. 3CLpro also inhibited platelet activation and aggregation in response to the platelet agonist thrombin.
Implications
These newly described extracellular functions of 3CLpro may promote the spread of SARS-CoV-2 infection to distal tissues, such as the brain, liver, kidneys, and heart.
Furthermore, this discovery of the regulated secretion of a viral protease with extracellular activity opens the door for future studies to investigate whether release of viral proteases from infected cells occurs with other coronaviruses or diverse virus families.
Source Publication: Grin, P. M., Baid, K., de Jesus, H. C., Kozarac, N., Bell, P. A., Jiang, S. Z., Kappelhoff, R., Butler, G.S., Leborgne, N.G.F., Pan, C., Pablos, I., Machado, Y., Vederas, J.C., Kim, H., Benarafa, C., Banerjee, A., & Overall, C. M. (2024). SARS-CoV-2 3CLpro (main protease) regulates caspase activation of gasdermin-D/E pores leading to secretion and extracellular activity of 3CLpro. Cell Reports, 43(12).