An international team of scientists has used mini-kidneys simulating those of diabetic patients to improve understanding of the link between diabetes and COVID-19. The researchers found that diabetic mini-kidneys have a higher susceptibility to SARS-CoV-2 infection than non-diabetic mini-kidneys.
The study, published in the journal Cell Metabolism, also identified genetic evidence for the essential role of the ACE2 receptor in COVID-19. A number of previous studies have indicated that people with diabetes are more likely to develop severe COVID-19, and that a number of patients hospitalized with COVID-19 suffer acute kidney damage. But to date, the molecular and metabolic mechanisms underlying these elements are not fully understood.
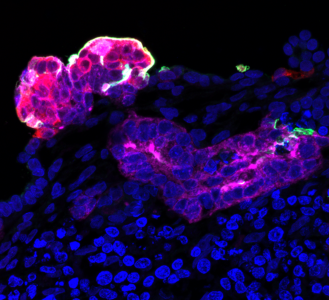
A high magnification detail of a a proximal tubular structure (magenta) within a kidney organoid after SARS-CoV-2 infection, that shows ACE2 positive cells (green) and SARS-CoV-2 infected cells (red)
Led by Professor Nuria Montserrat at the Institute of Bioengineering of Catalonia (IBEC) in Spain, a collaboration with UBC Life Sciences Institute Director Dr. Josef Penninger and researchers from the University of Florida, Karolinska Institutet and Karolinska University Hospital in Sweden developed kidney organoids (mini-kidneys) that simulate human conditions in the early stages of diabetes. Using molecular biology techniques including gene editing, the researchers observed that in diabetic mini-kidneys it is the abundance of the ACE2 receptor, an established gateway for SARS-CoV-2 viral entry, that determines susceptibility to viral infection.
“It is absolutely imperative to understand the molecular mechanisms that underlie more severe COVID-19 in patients with diabetes and other metabolic comorbidities,” says co-corresponding author Dr. Penninger.
Diabetic kidneys more prone to infection
Comparisons of kidney cells from patients with diabetes and individuals without the disease further demonstrated that kidney cells from diabetic patients had more ACE2 receptors and suffered a higher rate of SARS-CoV-2 infection.
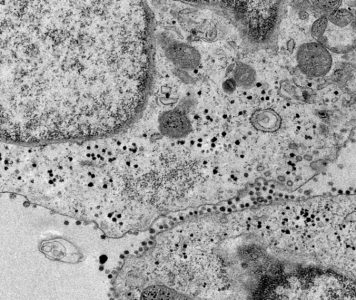
Transmission electron microscopy image showing a detail of kidney organoid cells infected with SARS-CoV-2.
“Our diabetic renal organoid model has allowed us to observe that diabetic mini-kidneys, with a greater number of ACE2 receptors, have a greater susceptibility to viral infection,” says Dr. Elena Garreta, a senior researcher from the Institute for Bioengineering of Catalonia and first co-author of the study.
“The development of a diabetic kidney organoid is a great step towards experimentally dissecting how metabolic changes can impact SARS-CoV-2 infections. The data again demonstrate that ACE2 is the essential receptor for SARS-CoV-2 even under conditions of comorbidity,” adds Dr. Penninger.
To delve into the mechanisms that may explain such observations, the researchers used a compound of substances that modulates the metabolic state of cells and found that the treatment reduced viral infection.
“We have shown that the SARS-CoV-2 virus is capable of directly infecting proximal tubule cells isolated from the human kidney and that diabetes makes these cells more prone to infection,” says Dr. Megan Stanifer an assistant professor at the University of Florida and first co-author of the study.
“This finding sheds light on a potential mechanism behind more severe cases of COVID-19 in diabetic patients. This technology will improve our capability to investigate how viruses interact with different organs in the human body,” concludes Dr. Ali Mirazimi, adjunct professor at the Department of Laboratory Medicine, Karolinska Institutet, and one of the study’s corresponding authors.
This research was supported in part by the Swedish Research Council, the European Research Council, the Institute of Health Carlos III and the Foundation Banco Bilbao Vizcaya, among others.
The authors have declared that a patent has been submitted to use human organoids to study SARS-CoV-2 infections and possibly develop new therapies. Josef Penninger is shareholder of Apeiron Biologics that is developing soluble ACE2 for COVID-19 therapy.
This story is based on a press release from the Institute of Bioengineering of Catalonia (IBEC) and an article from the Karolinska Institutet.
Publication
“A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells,” Elena Garreta, Patricia Prado, Megan Stanifer, Vanessa Monteil, Andrés Marco, Asier Ullate-Agote, Daniel Moya-Rull, Amaia Vilas-Zornoza, Carolina Tarantino, Juan Pablo Romero, Gustav Jonsson, Roger Oria, Alexandra Leopoldi, Astrid Hagelkruys, Maria Gallo, Federico González, Pere Domingo-Pedrol, Aleix Gavaldà, Carmen Hurtado del Pozo, Omar Hasan Ali, Pedro Ventura-Aguiar, Josep María Campistol, Felipe Prosper, Ali Mirazimi, Steeve Boulant, Josef M. Penninger, Nuria Montserrat. Cell Metabolism, online 12 maj, 2022, doi: 10.1016/j.cmet.2022.04.009