Gut infections cause misery that can reverberate throughout the enteric nervous system, leaving lasting tissue damage in their wake.
“We need to understand how to fix the mess that’s left behind,” says Dr. Lisa Osborne, an assistant professor in UBC’s Department of Microbiology and Immunology. “For intestinal infections, we know some of the common symptoms – it doesn’t necessarily matter which microbe is causing the infection, the result is pain and changes in gut motility (either constipation or diarrhea) that can last for days, weeks or in some unlucky cases, even months after the infection clears up. Why is that?”
A Nov 11 publication in Cell out of the lab of Daniel Mucida at The Rockefeller University and Howard Hughes Medical Institute provides some important indications. In an issue of Cell’s sister publication Neuron, Osborne was asked to asked to provide a commentary on what she describes as a “dense body of work.”
“It felt like the ‘prelude’ summarizing the other papers was necessary to put this one into context,” Osborne reflects.
A Q &A about the commentary
Why were you asked to comment on this very interesting paper?
I think I may have been chosen based on the marriage of two somewhat ’niche’ research areas, helminth infections/type 2 immunity and gut-brain interactions. Only a few papers have demonstrated a solid link between these two areas, so there’s a limited pool of experts who can speak to both. I’ve been studying type 2 immunity and regulation of host responses to helminth infections for years, and some of those studies revealed a role for alternative activation of macrophages (the stars of this show), and when I started my lab here was able to expand into gut-brain signaling. These interests gave me some perspective on the biology in question for this article, and I was happy to apply it.

Dr. Lisa Osborne
What are the main takeaways? We could start from the standpoint of your tweet text: “Intestinal infections are awful: everything hurts and sometimes things are ‘off’ for a while, even after the infection is cleared. Turns out, specialized macrophages play key roles in re-setting gut function, and can even help if you get re-infected.”
One area of research that is gaining more traction these days is recovery from infections. Unsurprisingly, a lot of time and money has gone into understanding the cells and molecular pathways that are involved in effective immune clearance of pathogens (or, when the immune system needs help, antibiotics, antivirals, antifungals etc). But microbial infections can cause a lot of tissue damage, and we need to understand how to fix the mess that is left behind. For intestinal infections, we know some of the common symptoms – it doesn’t necessarily matter which microbe is causing the infection, the result is pain and changes in gut motility (either constipation or diarrhea) that can last for days, weeks or in some unlucky cases, even months after the infection clears up. Why is that?
Earlier papers from the Mucida lab showed that neurons embedded in the gut wall can die off following Salmonella infection (Salmonella Typhimumium is the mouse version of Salmonella typhi, which in humans causes typhoid fever, a food-borne infection that can have devastating consequences. The mouse version doesn’t cause nearly as much trouble for mice, but is a good model for investigating intestinal responses to infection). Neuron death was associated with slowed intestinal transit time (mimicking constipation in people), and it took up to 4 months after the infection was cleared for neurons to come back and for the mouse gut to function properly. They also identified that alternative activation of a specific subset of macrophages that reside near these neurons can limit Salmonella-induced damage to the neurons – if they manipulated mice to deplete that macrophage subset, Salmonella infection resulted in more neuronal death and even slower gut movement.
This paper that the commentary was written about shows a few things: first, not all intestinal infections kill enteric neurons. That’s a good thing! But even cooler, even if neurons don’t die off, their neighbouring macrophages go through the same alternative activation and that alternative activation state is maintained for a while (up to 12-24 weeks). Second, when the investigators infected mice with either bacteria or parasitic worms, and then came back with a new Salmonella infection a few weeks later…the mice experienced far less death of gut-resident neurons and gut transit time stayed closer to normal. Importantly, the protection of enteric neurons and gut motility relied on alternative activation of this specialized subset of macrophages.
Overall, my interpretation of the data is that our tissues have developed mechanisms to cope with repeated infectious exposure. If one infection does happen, a subset of immune cells learns from it, and adopts a protective phenotype so that if we come in contact with a new microbe, the cells are more prepared to limit damage caused by the new infection.
In your final sentence, you point to a variety of new avenues for research. Is a particular path resonant with your own research program? What needs to come first?
A few years ago, we published a review about helminth infections and multiple sclerosis. MS is an autoimmune disease that results in neuronal damage, and in mice and rats at least, helminth infections can protect against MS-like disease. Observations of this protective effect have been around for close to 20 years. But what we learned while writing this review is that there is very little known about how helminth infections affect the central nervous system, which made us wonder whether there could be local effects that protect neurons. We’re actively pursuing this line of questioning, and this paper is just one more piece of data suggesting that alternative activation of macrophages might be important.
In a path that has nothing to do with our work, I’d really like to see pain measurements in their models. Gut pain can be crippling, and if the macrophages they’ve identified that reside in the gut wall next to neurons have anything to do with how pain is perceived, they could represent a new therapeutic target.
What are the implications of these findings for people with IBS, and their clinicians?
So far, I think it’s at the level of interesting findings. In order for this to have translational value, we’d need studies investigating neuronal health and macrophage function in people with IBS. Are these macrophage subsets less likely to be alternatively activated in people with IBS than the rest of the population? That might be the place to start, and build from there.
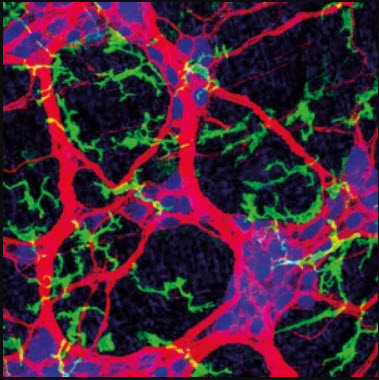
Image: Confocal microscopy image of the myenteric plexus isolated from naive wild-type mouse showing enteric-associated neurons (red) and MMs (green) in close association. Courtesy of I. Gabanyi, P. A. Muller and D. Mucida.
Read the Papers
Ahrends T, Aydin B, Matheis F, Classon CH, Marchildon F, Furtado GC, Lira SA, Mucida D. Enteric pathogens induce tissue tolerance and prevent neuronal loss from subsequent infections. Cell. 2021 Nov 11;184(23):5715-5727.e12. doi: 10.1016/j.cell.2021.10.004. Epub 2021 Oct 29. PMID: 34717799; PMCID: PMC8595755.
Osborne LC. Protecting your gut feelings: How intestinal infections keep things moving. Neuron. 2021 Nov 17;109(22):3545-3547. doi: 10.1016/j.neuron.2021.10.037. PMID: 34793706.