To honour the 30th anniversary of the journal Bioconjugate Chemistry, Dr. Pieter Cullis and collaborators from UBC and the Netherlands published their first review article showcasing the potential of gene therapy and lipid nanoparticles (LNPs) in treating diseases by gene silencing, protein expression, or gene correction.
To efficiently deliver the nucleic acid payload to its target tissue, say the authors, the genetic material needs to be combined with a delivery platform. Lipid nanoparticles (LNPs) have proven to be excellent delivery vectors for gene therapy and are increasingly entering into routine clinical practice.
Dr. Cullis and colleagues summarize recent discoveries about the biomolecular corona, expanding the knowledge gained with other nanoparticles to LNPs for nucleic acid delivery. In particular, they address how particle stability, biodistribution, and targeting of LNPs can be influenced by the biological environment. Onpattro is used as a case study to describe both the successful development of an LNP formulation for gene therapy and the key influence of the biological environment. The authors also outline the techniques available to isolate and analyze the corona of LNPs, and highlight their advantages and drawbacks.
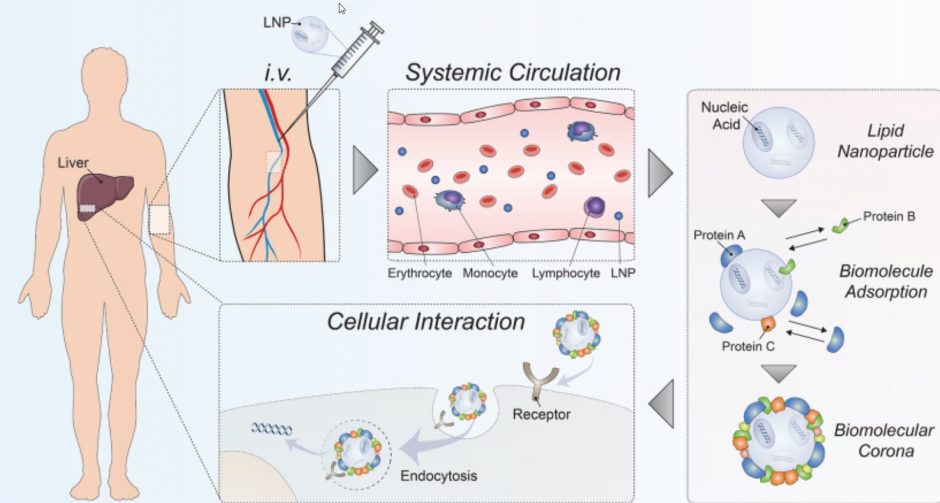 Over the past two decades, the optimization of LNP formulations for nucleic acid delivery has led to a well-established body of knowledge culminating in the first-ever RNA interference therapeutic using LNP technology—Onpattro—and many more in clinical development to deliver various nucleic acid payloads. Screening a lipid library in vivo for optimal gene silencing potency in hepatocytes resulted in the identification of the Onpattro formulation. Subsequent studies discovered that the key to Onpattro’s liver tropism is its ability to form a specific “biomolecular corona”.
Over the past two decades, the optimization of LNP formulations for nucleic acid delivery has led to a well-established body of knowledge culminating in the first-ever RNA interference therapeutic using LNP technology—Onpattro—and many more in clinical development to deliver various nucleic acid payloads. Screening a lipid library in vivo for optimal gene silencing potency in hepatocytes resulted in the identification of the Onpattro formulation. Subsequent studies discovered that the key to Onpattro’s liver tropism is its ability to form a specific “biomolecular corona”.
The authors discuss possible implications of the biomolecular corona for LNP delivery and examine the potential of exploiting the corona as a targeting strategy beyond the liver to develop next-generation gene therapies. For example, Apolipoprotein E (ApoE), among other proteins, adsorbed to the LNP surface enables specific hepatocyte targeting. This proof-of-principle example demonstrates the use of the biomolecular corona for targeting specific receptors and cells, thereby opening up the road to rationally designing LNPs. To date, only a few studies have explored in detail the corona of LNPs, and how to efficiently modulate the corona remains poorly understood.
Image: PEG lipids can be incorporated into LNPs conferring them “stealth” properties. The lipid chain length of PEG alters LNP blood circulation and biodistribution.
Read the Paper
Francia, V. Schiffelers R.M., Cullis, P.R., Witzigmann, D. The Biomolecular Corona of Lipid Nanoparticles for Gene Therapy. Bioconjugate Chem. [online 2020]
DOI: 10.1021/acs.bioconjchem.0c00366
