New Research from the Van Petegem lab published in Molecular Cell shows that stress signals can result in heart arrhythmia even in the absence of genetic predisposition to arrhythmias
When our heart muscles need to contract, calcium ions play a special role. The Sarcoplasmic Reticulum (SR) is a specialized organelle that forms a large intracellular supply of Ca2+. They are released from the SR through a specialized ion channel with a massive size of 2.2MDa. Also known as the Ryanodine Receptor (RyR), this channel is of key importance in muscle contraction, and is the target for hundreds of disease-causing mutations that cause arrhythmia, muscle weakness, and a dan
gerous reaction to certain anesthetics.
This channel is also under control of stress signals. When beta-adrenergic receptors are activated, this leads to activation of kinases (PKA, CaMKII) that can phosphorylate the RyR. This then facilitates opening of the channel, such that Ca2+ is released more readily. In normal circumstances, this can help with the increased cardiac output during exercise or stress in general, but when excessive, the phosphorylation of RyRs can directly contribute to heart rhythm disorders, muscle fatigue, and much more.
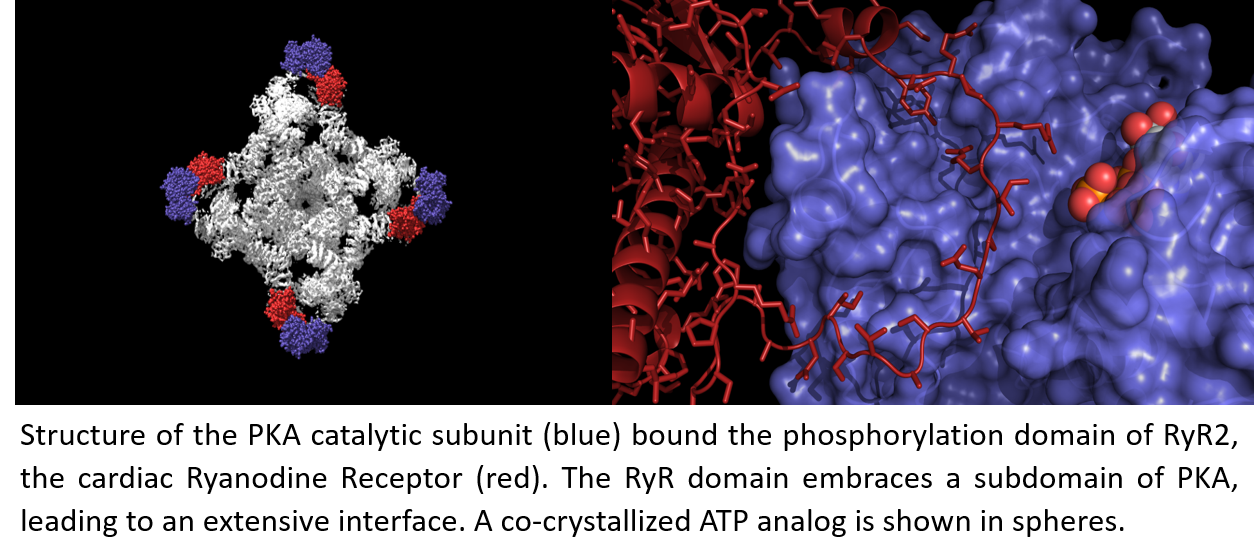
In this study, Dr. Haji-Ghassemi, a postdoctoral fellow in the lab of Dr. Filip Van Petegem determined high-resolution crystal structures of PKA bound to its interaction domain in the RyR. A major surprise is that the interface is much larger than previously anticipated, with a large loop of the RyR forming a ‘lasso’ around a subdomain of PKA. The interface is the target for many mutations that cause CPVT, a severe type of cardiac arrhythmia. In addition, the team looked at the impact of phosphorylation of the RyR by CaMKII and found that this increases the efficiency of the PKA enzyme. High-resolution structures show that the phosphorylation results in structural changes, including the formation of a new α-helix, which in turn increases the affinity of PKA for the RyR. There is thus a direct molecular interplay between PKA and CaMKII on RyRs.

Graphical Abstract
Read Canadian Light Source Press Release: https://www.lightsource.ca/news/details/stress_can_lead_to_heart_failure.html
Read Full Publication: https://www.cell.com/molecular-cell/fulltext/S1097-2765(19)30311-9
For more on research in the Van Petegem Lab: http://crg.ubc.ca/VanPetegem/index.html